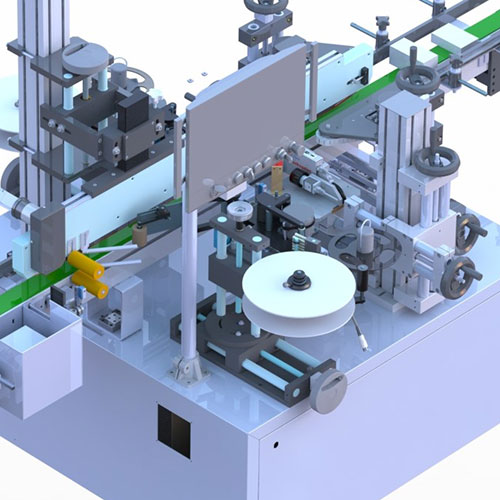
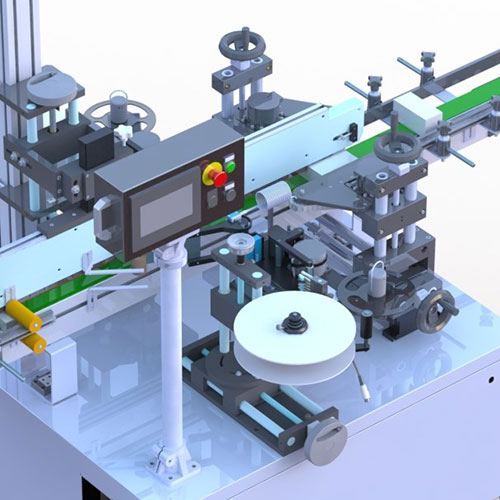
Pharmatronik solutions can be customized to enterprise specific needs in order to ensure the utmost flexibility, future expandability and full compliance with evolving requirements.
For all our machines we can offer Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ), an essential part of quality assurance through equipment validation. A further guarantee for your right choice.
What Do IQ, OQ and PQ Mean?
IQ OQ PQ protocols are ways of establishing that the equipment which is being used or installed will offer a high degree of quality assurance, so that manufacturing processes will consistently guarantee products that meet predetermined quality requirements. To better understand these terms, let’s look at them one by one:
Installation Qualification (IQ)
Any new equipment is first validated to check if it is capable of producing the desired results through Design Qualification (DQ), but its performance in a real-world scenario depends on the installation procedure that is followed. Installation Qualification (IQ) verifies that instruments or equipments being qualified, as well as their sub-systems and any ancillary systems, have been delivered, installed and configured in accordance with the manufacturer’s specifications or installation checklist.
Operational Qualification (OQ)
Once each protocol of the IQ phase has been met, Operational qualification (OQ) is performed to check that the equipment’s performance is consistent with the user requirement specification, within the manufacturer-specified operating ranges. During the OQ phase, all the items in the test plan are tested individually and their performance documented.
Performance Qualification (PQ)
Performance Qualification (PQ) is the final step in the qualification process for equipment, and involves verifying and documenting that the equipment is working reproducibly within a specified working range. Rather than testing each instrument individually, they are all tested together as a part of an overall process. Before the qualification begins, a detailed test plan is created, based on the process description.